Combining electricity and water might not sound like the most intelligent thing to do, but when electricity is used correctly it can be a helpful tool in wastewater treatment. Electricity has been used for water treatment for the past few decades, but limitations in technology and high operating costs compared to other treatment processes have prevented its wide-scale use as a major wastewater treatment method. However, the tightening of environmental restrictions for wastewater discharge has brought a new drive to develop electrochemical water treatment (EWT) processes.
The basic principle behind EWT processes is very simple: electrodes are used to apply an electrical current to water to get the required reaction. Despite its simplicity, the result can be quite hard to predict as the process is a mixture of water and electrochemistry.
Different types of EWT
EWT processes can be roughly divided into three different types: electro-coagulation (EC), electro-flotation (EF), and electro-oxidation (EO). However, there is no clear differentiation between the types and some authors use the terms interchangeably, meaning extra attention must be paid when reading relevant literature.
Electro-coagulation, where process electricity is used to dissolve metal from a sacrificial electrode (anode) into the treatment water, is probably the most well known of these methods. The dissolved metal then reacts with both the impurities in the water and the water itself, which causes coagulation. The treatment with EWT does not add salts to the water as conventional precipitation processes do. The most common electrode materials for EC are iron and aluminum because of their proven efficiency, affordable price, and wide availability.
In electro-oxidation, an inert electrode coated, for example, in titanium or boron-doped diamond is used as the anode. In EO the dissolution reaction is replaced by oxygen formation from the electrical dissociation of water. In some cases, chlorine might be formed, but it readily reacts to hypochlorite that acts as a strong oxidant making the process more efficient.
The gases generated by the dissociation of water on the electrodes during EWT can be used to float either the formed particles in the case of EC or other impurities in the water. This is called electro-flotation. Usually EF is seen as part of EC or EO, but it can also be implemented as its own process.
Target applications for EWT
Recently EWT processes have been studied in various industries and with various different pollutants. The most common applications studied are the removal of metal ions/oxyhydroxides, oil removal, and the removal of organic matter. In general, when compared to traditional precipitation processes, EWT technology gets more competitive as concentrations get lower.
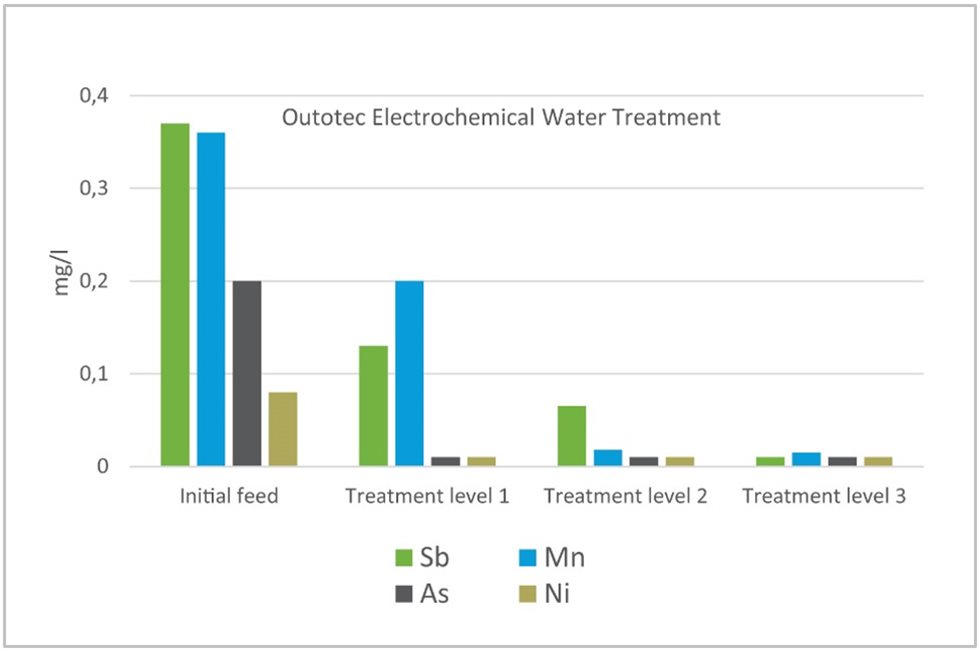
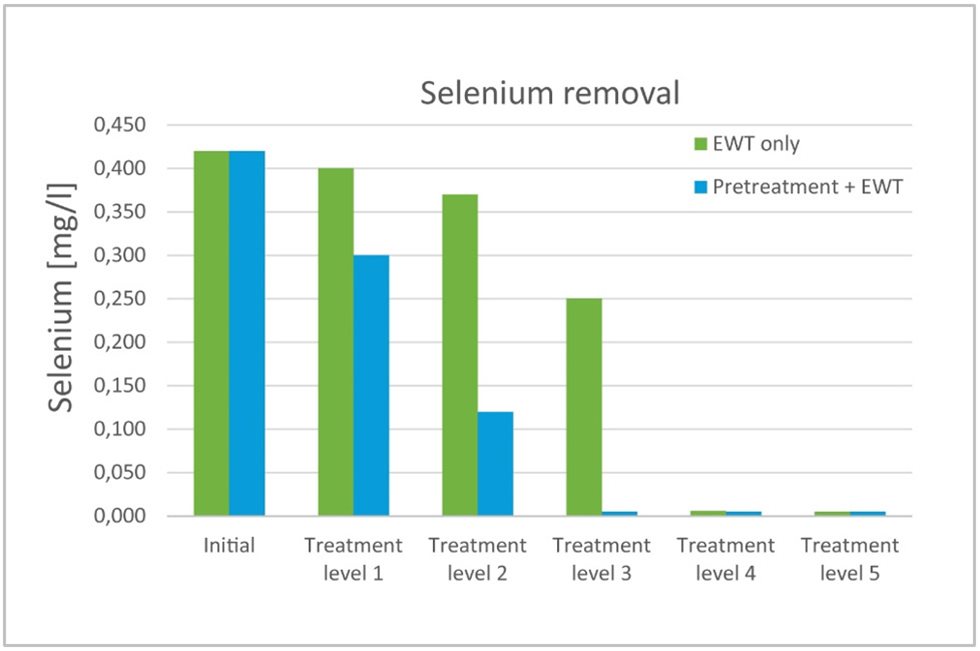
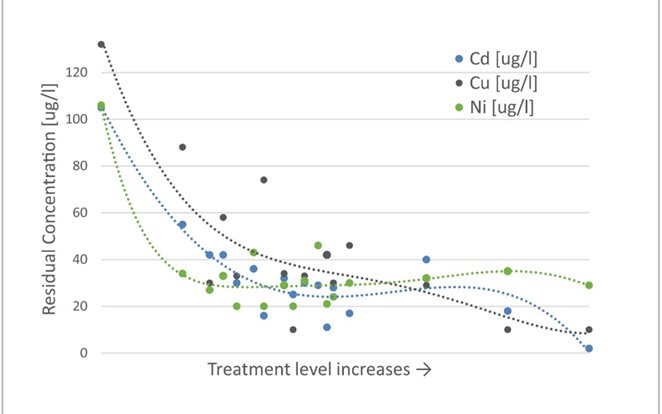
The biggest potential of EWT technology is in the wastewater polishing process for substances that are hard to remove using traditional precipitation processes, such as oxyanions like arsenic, antimony, and selenium, or metal residuals like cadmium, nickel, and copper.
Factors affecting EWT
The main factors affecting EWT are current density or applied current, and residence time in cell or flowrate. Current density [A/m2] is the amount of current applied to a cross-sectional area of the electrode and is the main factor that defines which electrochemical reactions take place on the electrode surface. It also determines the rate of electrode dissolution, bubble generation, and potential in the cell, so it greatly affects the economics of the treatment.
Charge loading [C/m3, As/m3] defines the amount of electricity applied to an electrolyte by volume. It is directly proportional to the amount the electrode has dissolved, i.e. it states the dosage of the metal. Charge loading consists of the applied current and flowrate, thus combining both the main parameters of the process. It can also be said that charge loading is the treatment level of the process.
EWT technology has been proven to work in a wide temperature and pH range. Furthermore, the EWT process naturally drives the pH towards a slightly alkaline (pH 8-9) level, neutralizing the solution and usually allowing a direct discharge. One good thing to remember when designing an EWT system is that aluminum electrodes can be passivated more easily at certain pH levels, whereas iron electrodes are more flexible.
The composition of the water under treatment and its purification needs determine the final level of applied current and flowrate. It is therefore important to have a definite target in mind so the EWT process can be designed and optimized. In addition, ionic concentrations (i.e. impurities) affect the conductivity of the water, determining the system voltage and the treatment cost.
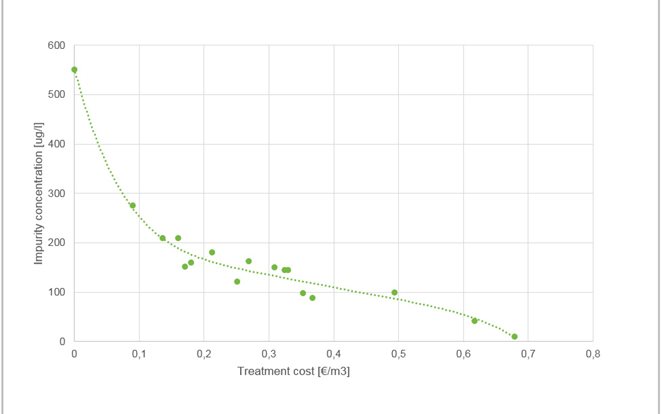
EWT results and costs
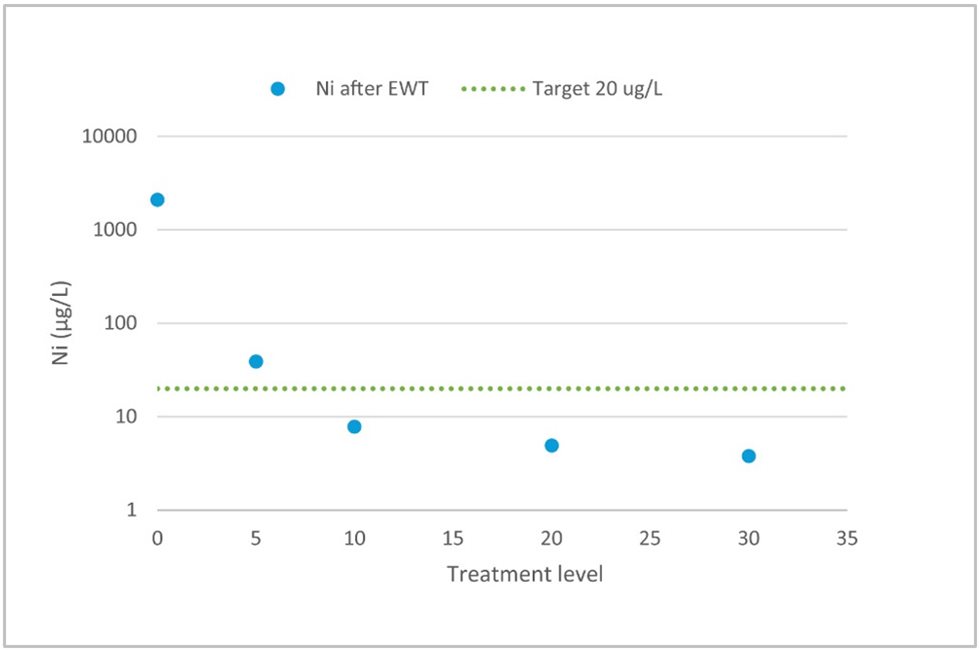
In all of the following examples, treatment level means charge loading. As previously mentioned, the EWT process works most efficiently as a polishing process. If the initial concentration of impurities is less than 10 mg/l we can expect treated water to reach residual levels of 20 μg/l. Figure 2 gives an example of water that is well suited for EWT technology. Initially, there weren’t many impurities in the water, but the discharge limit was as low as 20 μg/l for nickel.